The elements
In 1661 Robert Boyle discovered the rules of mixing elements in constant proportions, and proposed a new way to look at matter, replacing the ancient idea of the classical elements (fire, air, water, earth) and the ideas of the alchemists. In 1789 Antoine Lavoisier listed 33 elements including light and caloric in his book "Elements of Chemistry". By 1818 Jons Jakob Berzelius had found the atomic weights of 45 of the 49 known and accepted elements. In 1869 Dmitri Mendeleev arranged sixty-six elements in the periodic table accordind to their weight and properties. In this way he was able to predict the weight and properties of some missing elements.
| 1661 | Robert Boyle discovers the mixing of elements in constant proportions. |
| 1789 | Antoine Lavoisier listed 33 elements including light and caloric in his book the Elements of Chemistry. |
| 1818 | Jons Jakob Berzelius found the atomic weights of 45 of the 49 known elements. |
| 1869 | Dmitri Mendeleev arranged 66 elements in the periodic table predicting the existance of the elements unknown at the time. |
Elements are meant to represent the smallest components of matter, not capable of being divided into small components. Further discoveries found that these elements are in fact made up of more fundamental particles, explaining the existence of different isotopes of the same element. Since the element of the periodic table cannot be broken down by chemical means, it is correct to refer to them as the chemical elements.
Periodic table of the elements
It is thought that the elements were forged by nuclear reactions within long extinct stars, and that this explains the chemical abundance of the different elements in the universe.
To hear a great song about the elements, search for "Meet The Elements - They Might Be Giants ".
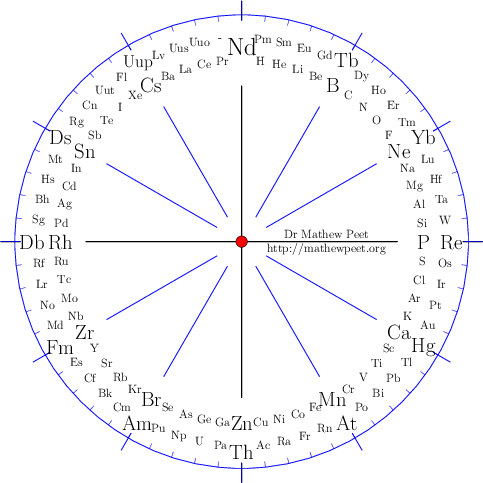
Download Chemical Elements Clock as a pdf. The elements are listed by atomic number, with 60 divisions around the clock face. It is easy to read the atomic number by the position.