| ( |
| ( |
The possibility that the thermal cycling of supercooled austenite, within the bainite transformation range, accelerates the kinetics of decomposition is investigated both by analysing previous work and by conducting a series of new experiments. The cyclic variations in temperature do not seem to anomalously affect the rate of transformation.
There is a novel range of steels under development, based on bainite forming at exceptionally low temperatures [1–6]. The advantage of suppressing the transformation temperature is that incredibly fine platelets of bainitic ferrite are generated which are only 20–40 nm in thickness. This makes the steel strong and in combination with the austenite that is retained, gives a useful range of properties which have been exploited in the manufacture of armour [7, 8]. There are other applications in the aircraft industry that are at a critical stage of development.
One characteristic of transformation at low temperatures is that the rate of reaction is slow, sometimes taking days in order to achieve the maximum fraction of bainitic ferrite. The reaction can be accelerated by refining the austenite grain size and by adding solutes which increase the free energy change accompanying transformation [9].
Sista et al. have suggested an alternative method, that the reaction can be speeded up by cycling the austenite between two temperatures within the range MS–BS, the martensite and bainite–start temperatures respectively [10]. Single step–changes in the temperature during the course of the reaction has been investigated before [11], to show indirectly that the stresses generated by phase transformation at low temperatures can stimulate transformation at higher temperatures.
As will be described later, there are some difficulties in interpreting published data. The purpose of the present work was therefore to investigate the influence of thermal cycling on the kinetics of the low–temperature bainite reaction. The paper begins with an assessment of the previous work [10] and goes on to present and interpret new experiments.
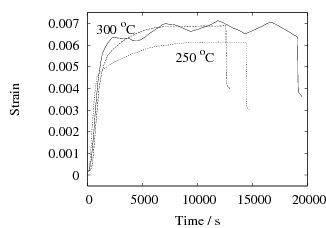
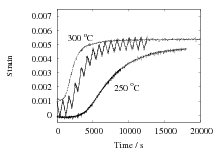
In their experiments on SAE 1080 steel, Sista et al. [10] supercooled the austenite into the bainite transformation range, and cycled the temperature linearly between 260–300∘C. It was claimed that the cycled samples formed bainite much more rapidly than those which were simply isothermally held at 260 or 300∘C. The data were presented as separate graphs from each experiment and hence were difficult to correlate. The digitised data are replotted in Fig. 1 for the two cases reported.
A close examination of the data for the low time periods where most of the reaction is observed, shows that the cyclic treatment did not in fact increase the rate of transformation relative to isothermal transformation at 300∘C. In both cases, the cyclic curve was, as might be expected, enveloped between the two isothermal transformation curves. The extracted data in Table 1 confirm the conclusion that there is no evidence for an acceleration of transformation due to the thermal cycling. Note that there is also a difficulty with the design of the experiments because in both cases, almost all of the transformation is completed before the first cycle is applied.
| 5% time / s | 95% time /s | |
| Isothermally transformed at 260∘C | 420 | 4600 |
| Isothermally transformed at 300∘C | 180 | 5400 |
| Cyclic treatment (80 min period) | 300 | 5700 |
| Cyclic treatment (16 min period) | 370 | 6000 |
The steel used belongs to the class of low–temperature bainite [1–6, 12] but with cobalt and aluminium added in order to reduce the transformation time from days to hours [9, 13]. This is necessary in order to conduct experiments on a thermomechanical simulator. The chemical composition of the steel is given in Table 2.
Cylindrical samples 8 mm diameter and 12 mm length were austenitised at 1000∘C for 15 min followed by cooling to the isothermal transformation temperature, or for a variety of cyclic heat treatments. All the heat treatments were carried out in a Thermecmastor thermomechanical simulator which has been described elsewhere [9]. The results were supported with routine optical microscopy and Vickers hardness measurements.
| C | Si | Mn | Mo | Cr | Co | Al |
| 0.78 | 1.6 | 2.02 | 0.25 | 1.01 | 3.87 | 1.37 |
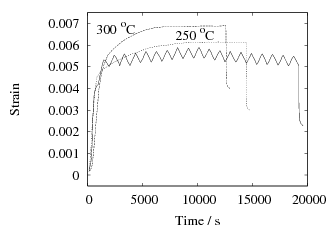
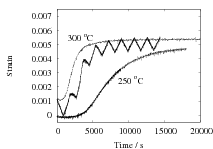
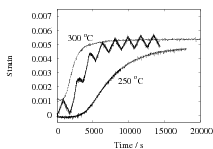
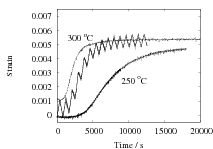
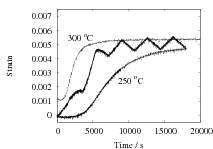
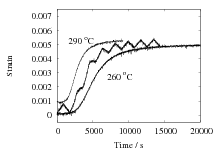
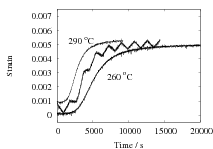
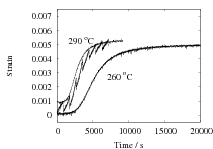
The results are presented in Fig. 2 for both isothermal, and cyclic treatments, where the latter are said to be symmetrical since the magnitudes of the heating and cooling rates were identical within each cycle. The diameter of each sample on reaching the designated temperature is plotted so that the vertical distance between any two curves at the beginning of the plot, corresponds exactly to the strain due to thermal contraction alone. All of the data show that cyclic transformation does not lead to an acceleration of the reaction. Note also that the transformation rates are such that much of the decomposition of austenite takes place during the cycling. Further confirmatory results are shown in Fig. 3, including one which has an asymetrical cycle.
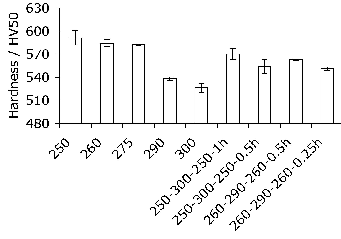
Figure 4 shows Vickers hardness data – mean values from five measurements in each case are plotted along with their standard deviations. The hardness is a good indicator of the progress of transformation at long times [5]. It is evident that in all cases, the hardness of the cycled samples falls between that of the bounding isothermal tests.
We do not find that alterations of temperature within the bainite transformation range perceptibly affect the reaction rate when compared with isothermal reaction.
| ( |

| ( |

| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
The authors are grateful to British Universities Iraq Consortium for funding this work, to the Ministry of Education in Iraq and to the University of Cambridge for the provision of laboratory facilities.
[1] F. G. Caballero, H. K. D. H. Bhadeshia, K. J. A. Mawella, D. G. Jones, and P. Brown. Very strong, low–temperature bainite. Materials Science and Technology, 18:279–284, 2002.
[2] F. G. Caballero and H. K. D. H. Bhadeshia. Very strong bainite. Current Opinion in Solid State and Materials Science, 8:186–193, 2005.
[3] F. G. Caballero, H. K. D. H. Bhadeshia, K. J. A. Mawella, D. G. Jones, and P. Brown. Design of novel high–strength bainitic steels: Part i. Materials Science and Technology, 17:512–516, 2001.
[4] F. G. Caballero, H. K. D. H. Bhadeshia, K. J. A. Mawella, D. G. Jones, and P. Brown. Design of novel high–strength bainitic steels: Part ii. Materials Science and Technology, 17:517–522, 2001.
[5] C. Garcia-Mateo, F. G. Caballero, and H. K. D. H. Bhadeshia. Development of hard bainite. ISIJ International, 43:1238–1243, 2003.
[6] C. G. Mateo and H. K. D. H. Bhadeshia. Nucleation theory for high–carbon bainite. Materials Science and Engineering A, 378A:289–292, 2004.
[7] H. K. D. H. Bhadeshia. Large chunks of very strong steel. Materials Science and Technology, 21:1293–1302, 2005.
[8] P. M. Brown and D. P. Baxter. Hyper–strength bainitic steels. In Materials Science and Technology 2004, pages 433–438, Warrendale, Pennsylvania, USA, 2004. TMS.
[9] C. Garcia-Mateo, F. G. Caballero, and H. K. D. H. Bhadeshia. Acceleration of low–temperature bainite. ISIJ International, 43:1821–1825, 2003.
[10] V. Sista, P. Nash, and S. S. Sahay. Accelerated bainitic transformation during cyclic austempering. Journal of Materials Science, 42:9112–9115, 2007.
[11] R. H. Goodenow, R. H. Barkalow, and R. F. Hehemann. Bainite transformations in hypoeutectoid steels. In Physical properties of martensite and bainite, special report 93, pages 135–141, London, 1969. Iron and Steel Institute.
[12] H. K. D. H. Bhadeshia. Properties of fine–grained steels generated by displacive transformation. Materials Science and Engineering A, 481–482:36–39, 2008.
[13] H. I. Aaronson, H. A. Domian, and G. M. Pound. Partitioning of alloying elements between austenite and proeutectoid ferrite and bainite. TMS–AIME, 236:781–796, 1966.